  |
|
 |
 |
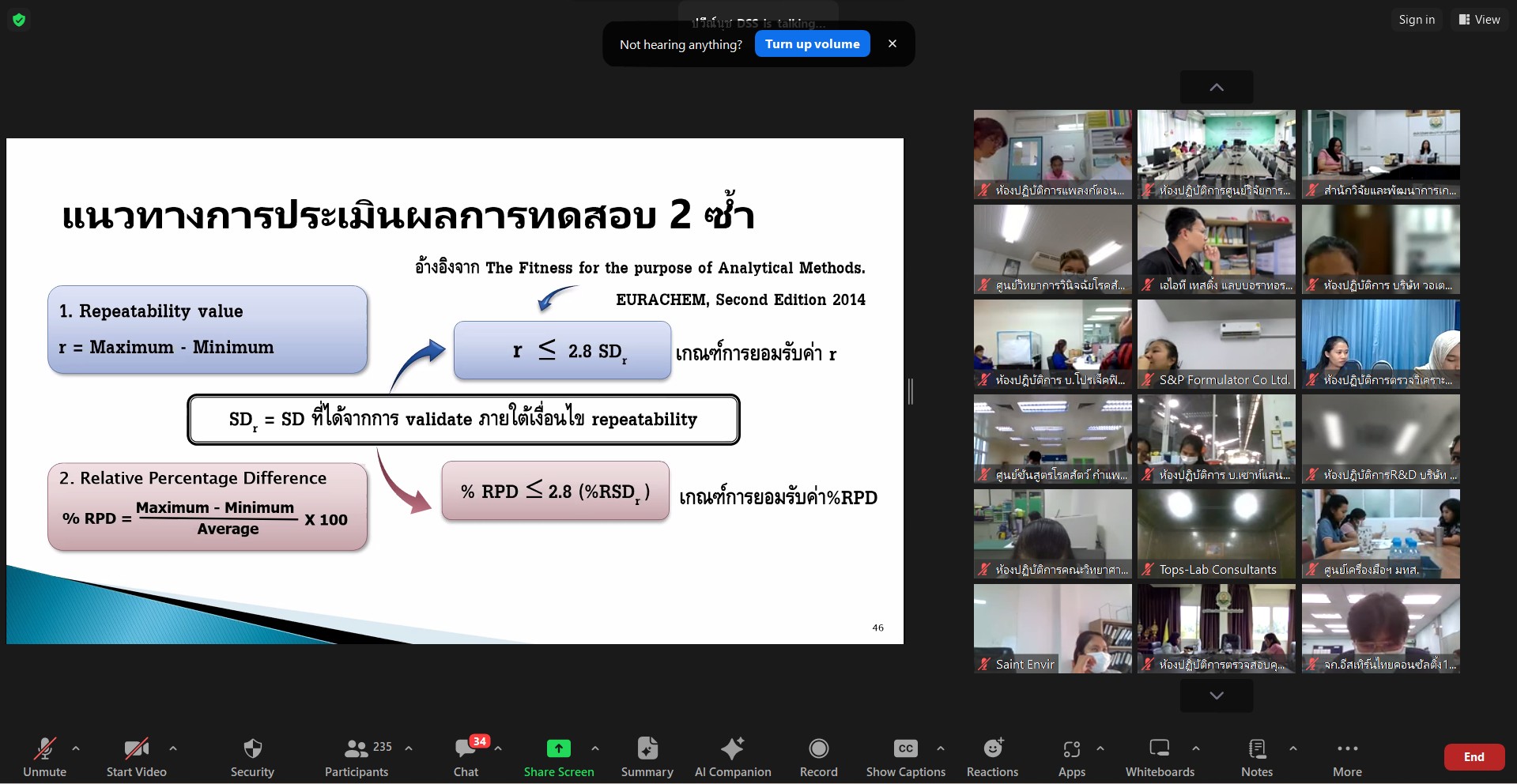
On February 27, 2024, the Bureau of Laboratory Accreditation, Department of Science Service (BLA-DSS) provided a virtual seminar “Strengthening Competence of Accreditation Bodies and Quality Assurance for International Recognition” to relevant stakeholders, with a use of Zoom Application. The BLA Director, Mrs. Chantarat Vorasapavit, reported the rationale and objectives of the seminar to the General Director, Rungrueng Kitphati. She reported, “the seminar is to enhance comprehensions and knowledge regarding method validation and enable attendees to integrate understanding into daily operations, with more effective and correct manners according to ISO/IEC 17025 and strive for high-level competence of laboratory testing in response to market needs. Mrs. Chantarat Vorasapavit also added that the seminar is one of the training and seminar plans set out by the BLA. This seminar will contribute to maximizing potentials of public and private sectors and their stakeholders according to given international standards, aiming to thrive testing laboratories to be accredited according to ISO/IEC 17025 and to fulfil the needs of business sectors which promote products ‘service and quality, subsequently.
The General Director, Dr. Rungrueng Kitphati, announced that BLA-DSS has committed to making significant changes in laboratory accreditation according to ISO/IEC 17011:2017 Conformity assessment-Requirement for accreditation bodies conformity assessment bodies. The body is responsible for laboratory accreditation with accordance with ISO/IEC 17025, which the specifications of the standard highlighting an establishment of confidence on “ensuring the validity of result”. Hence, quality assurance is needed to reach such level of confidence made testing results valid and widely recognized by international and national stakeholders. With this concern, BLA, an accreditation body, seeks to establish confidence and improve its competence according to laboratory accreditation to be more recognized at global level.
There were more than 300 attendees joined this seminar.


